What is a Flow Cytometry Panel?
What is a Multicolor Flow Cytometry Panel? It’s a list of read-outs (cell markers: intracellular or extracellular antigens) and/or cell characteristics (cell cycle stage, viability, proliferation…) that are simultaneously quantified and analyzed on single cells using a flow cytometer.
Each cell marker or characteristic is either targeted by an antibody that is fluorescently labeled (called fluorochrome-conjugated antibody) or by a fluorescent dye (such as a fluorescent protein, reactive dye that specifically binds to DNA or to cell organelles).
Flow cytometry panel
Generally, each read-out is correlated to a single different fluorochrome or fluorescent dye (except for markers associated to the same fluorochrome, known as dump channel markers).
Each marker is therefore detected and quantified through the collection of fluorescence emitted from its corresponding fluorochrome (also called “color”).
Increasingly large multicolor flow cytometry panels (of up to more than 50-60 fluorochromes/dyes) can now be run thanks to advances in optics, instrumentation and flow cytometry reagents, including the advent of spectral flow cytometry.
What is a Multiparametric Flow Cytometry Panel?
The reason multiparametric flow cytometry is attractive lies in the potential of simultaneously getting multiple read-outs from single cells, which allows deeper understanding of cell biology including more accurate definition of cell subpopulations and profiles. This entails the need for fewer samples and/or smaller number of cells, which is key for example for cells extracted from patient tissues or samples.
On the flip side however, running more antigens/fluorochromes increases the complexity of the experimental design, requiring more attention in fluorochrome selection and panel design.
Selecting the right combination of fluorochromes is indeed one of the biggest challenges in multiparameter flow cytometry and is mainly due to:
-the limited number of fluorochromes that are commercially available and that can be conjugated to antibodies
-the fact that different fluorochromes may be excited by the same laser (at the same wavelength) and may have overlapping emission spectra. This could lead to false positive signals since the measured signal may originate from different fluorochromes at the same time, i.e., fluorochromes not corresponding to the target read-out (since one read-out is generally traced back to one fluorochrome).
While there are some excellent resources and tools available online that guide multiparametric flow cytometry panel design, EasyPanel is the only one that actually suggests concrete optimized panels (based on users’ inputs).
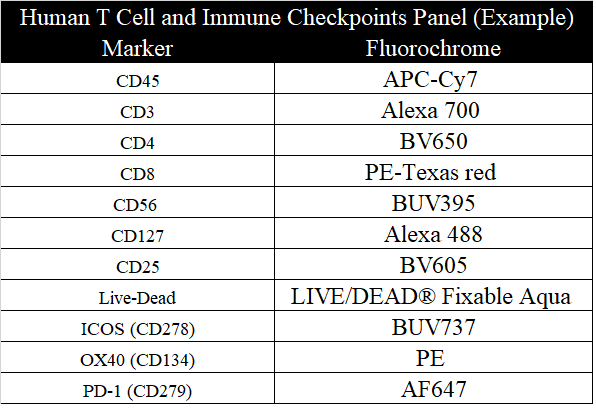
About EasyPanel
EasyPanel is an Automated and Intelligent tool for flow cytometry panel design (traditional and spectral cytometers). It is licensed by leading biotech, pharma and academic flow core facilities to design optimized panels of up to 48 colors in seconds or minutes.
Check us out and create a free demo account at: Click Here
 Explore the Scientific R&D Platform
Explore the Scientific R&D Platform 











